Study conducted by Henri Malaize (1.2) · Thomas Samoyeau (2.3) · Marc Zanello (1.2.4) · Alexandre Roux (1.2.4) · Joseph Benzakoun (2.3.4) · Sophie Peeters (5) · Gilles Zah - Bi (1.2) · Myriam Edjlali (2.3.4) Arnault Tauziede - Sponsorship (2.6) · Edouard dezamis (1.2) · Eduardo Parraga (1.2) · Fabrice Christian (2.6) · Pascale Varlet (2.4,6) · Geneviève Plu - Bureau (7.8) · Catherine Oppenheim (2.3,4) · under the direction of Johan Pallud (1.2.4)
Object
Improving knowledge on interactions between meningiomas and progestins makes it possible to refine the management of this specific state. We have evaluated the evolution over time of the management of meningiomas associated with a progestogen.
Methods
We have retrospectively studied consecutive adult patients who had at least a meningioma in the context of the taking of progestin ( October 1995-October 2018 ) in a university hospital center of adult neurosurgery.
Results
71 adult women with 125 meningiomas associated with a progestogen were included. The number of patients with meningiomas associated with a progestogy has increased over time (0.5/year before 2008, 22.0/year after 2017) . Progressive treatment was an indication validated in 27.0 % of cases. An average of 1.7 ± 1.2 meningiomas were discovered per patient (median 1, fork 1-6). Surgery was practiced out of 36 (28.8 %) meningiomas and the histopathological classification was grade WHO 1 for 61.1 % and grade 2 for 38.9 % . The conservative management of meningiomas increased with time (33.3 % before 2008, 64.3 % after 2017) and the withdrawal of progestin treatment increased with time (16.7 % before 2008, 95.2 % after 2017). Stopping treatment varies according to the progestin derivative used (88.9 % with cyproterone acetate, 84.6 % with chlormadinone acetate, 28.6 % with nomestrol acetate, 66.7 % with the combination of progestin derivatives). The main reason for the therapeutic management of meningiomas was the presence of clinical signs . Among the 54 meningiomas managed conservatively for which the progestin had been arrested, monitoring by MRI showed regression in 29.6 % of cases, stability in 68.5 % of cases and continuous growth in 1.9 % of cases.
Conclusions
Conservative management, including stopping progestin treatments, has developed over time with promising results in terms of efficiency and safety.
Keywords: Chlormadinone acetate; Cyproterone acetate; Meningioma; Neurosurgery; Nomestrol acetate; Progestogen.
Details of the AMAVEA
Cyproterone Actorate : Androcur
Acetate of Chlormadinone : Lotéran
Acetate of Nomestrol : Lutényl
1 Department of Neurosurgery, neurosurgery service, GHU Site Sainte-Anne, Paris, France
2 University of Paris, Paris, France
3 Department of Neuroradiology, GHU Site Sainte-Anne, Paris, France
4 Institut de Psychiatrie et Neurosciences de Paris (IPNP), UMR S1266, Inserm, Im-Brain, Paris, France
5 Department of Neurosurgery, University of California, Los Angeles, Los Angeles, CA, USA
6 Department of Neuropathology, GHU Site Sainte-Anne, Paris, France
7 Obstetricical, Perinatal and Pediatric Epidemiology Research Team, Center for Epidemiology and Biostatistes, Inserm U1153, Paris-Descartes University, Paris, France
8 Endocrinology Unit gynecology, University Hospital Paris Center, Cochin Hospital, Aphp, Paris, France
Introduction
Méningiomes are one of the primary tumors of the most frequent central system in adults [1]. Their incidence is higher in women (post-pubertal masculinity report of 2/1; 3.15/1 during the years of strong reproduction) [2].
The long-term use of large cumulative doses of progestogen (that is to say a synthetic derivative of progesterone having an anti-diand and progestogen activity), including cyproterone avatate, chlormadinone acetate and nomestrol acetate, is known to promote the development of meningiomes [3–10].
In France, cyproterone acetate is indicated for the treatment of advanced prostate cancer, reducing sexual impulses in paraphilies, and major female hirsutism [11]. Chlormadinone acetate is indicated for the treatment of menstrual disorders, symptoms of menopause, endometrial disorders hyperplasia and endometriosis [12]. Nomestrol acetate is indicated for the treatment of menstrual disorders, premenopausic disorders, functional genital hemorrhages, and uterine fibroids [13]. The three drugs have been widely used outside the indications authorized for acne or female alopecia. The link between high cumulative doses of progestogen and the growth of meningioma has been established since 2007 [14] and spontaneous regression of meningiomas after deprivation of progestogen was reported in 2008 for chlormaudinone acetate [15], in 2010 for cyproterone acetate [16-20], and in 2019 for nomestrol [21].
These advances in the knowledge of the interactions between meningiomas and progestins have changed the neurosurgical management of this specific condition. This observational study at a single center reports a retrospective analysis of consecutive adult patients under progestogen treatment, carrying meningioma and oriented towards neurosurgery. We have evaluated: (1) the characteristics of meningiomas; (2) Management of progestin treatment and (3) the management of meningiomas.
Material and methods
Data source
We have examined our files of adult patients who hosted at least a meningioma in the context of the taking of progestin between October 1995 and October 2018 (that is to say before the screening policy recommended in France by the National Sdes Medicines and Health Products, namely the National Agency for the Safety of Medicines and Health Products) and managed in our tertiary neurosurgical center for adults. The terms of research used (in French) are: meningioma and/or meningioma and/or acetate and/or acetate and/or cyproterone and/or cyproterone and/or Androcur ® and/or nomestrol and/or nomigestrol and/or Lotényl® and/or lutenyl® and/or chlormadinone and/or luteran® and/or luteran® and Luteran®. This study received the necessary authorizations (IRB #1: 2020/08) of the Institutional Revision Commission (IRB00011687). The obligation to obtain informed consent was lifted for this retrospective observation study according to the legislation.
Data collection
The data was obtained from medical records . The patient and the characteristics associated with the progestogen collected at the time of imaging diagnosis, are in particular: sex, age, symptoms at the time of diagnosis (asymptomatic, epilepsy crisis, increase in intracranial pressure pressure, neurological deficit), karnofsky performance status (kps), treatment with progestogen (molecule, reason for treatment, cumulative dose, Administration, the time interval between the treatment and diagnosis of meningioma), the characteristics of meningioma (number, location, volume, grade). The characteristics of treatment and monitoring included: monitoring duration, development of meningioma on imaging, management of each meningioma (conservative, surgery, radiotherapy), progestogen management (withdrawal of treatment or not), and complications related to treatment. All medical records were examined by a junior neurosurgeon (HM) and validated by a senior neurosurgeon (JP). All MRIs were examined by a junior neuroradiologist (TS) and validated by a senior neuroradiologist (JB).
Each dural lesion discovered by MRI and whose imaging characteristics are consistent with a meningioma on at least two successive MRIs was then called "meningioma", with or without histopathological examination confirmation . We divided the cohort on the basis of knowledge published at the time of the diagnosis of patients using the two benchmarks based on evidence: (1) 1 year after the first report of the growth of meningiomas after an estrogen -rogenic treatment in December 2007 [2, 14]; (2) 1 year after the first business series Demonstrate the stabilization of growth and the regression of meningiomas after stopping cyproterone acetate in October 2015 [18]. This method gave the following three results of time periods: from October 1995 to December 2008 (1995-2008), from January 2009 to December 2016 (2009-2016), and from January 2017 to October 2018 (2017-2018).
Statistical analyzes
Continuous variables have been described as a means ± standard deviation. The categorical variables have been described as percentages. Univocal analyzes have been carried out, by calculating the non-adjusted dimension reports and using the square CHI or the exact Fisher test to compare the categorical variables, and the unpaid T test or the Mann-Whitney-U test for the variables, if necessary. A probability value <0.05 was considered to be statistically significant. Statistical analyzes were carried out using JMP software (version 14.1.0; SAS Institute Inc, Cary, North Caryoline, United States).
Results
Population
In total, 71 patients (100 % women, 47 ± 10 years) were included. Clinical and imaging characteristics are detailed in Table 1. A single patient had already undergone a craniospinal radiotherapy treatment for a medulloblastoma. The number of patients with meningioma associated with a progestogen and supported in our establishment has increased over time: 0.5/year between 1995 and 2007, 9.2/year between 2008 and 2015, and 22.0/year between 2016 and 2018 (fig. 1).
Progestin treatment
The characteristics of the progestogy treatment are detailed in Table 1. Cyproterone acetate was administered in 47/71 cases (66.2 %), Chlormadinone acetate in 14/71 cases (19.7 %), nomestrol acetate in 71 (9.9 %), and a combination of progestin treatments in 3/71 cases (4.2 %) . The indication of the progestin treatment, available for 37/71 patients (52.1 %), was an indication approved in 10/37 cases (27.0 %). The prevalence of the prescription of unauthorized treatments has not changed over time (p = 0.240). The duration of the progestin treatment, available for 23/71 patients (32.4%), was 197 ± 71 months. The cumulative dose of progestin treatment, available for 6/71 patients (8.5 %), was 177.2 ± 89.1 grams. Time: the interval between the start of progestin treatment and meningioma diagnostic, available for 30/71 patients (42.3 %), was 202.0 ± 67.8 months. The time interval between meningiomas diagnosis and stopping progestin treatment, available for 41/71 patients (57.7 %), was 8.4 ± 17.2 months.
Characteristics of meningioma
The characteristics of meningiomas are detailed in Table 1. The reason for RM imaging was a clinically apparent symptom in 54/71 (76.1%) Case and screening in asymptomatic patients in 17/71 cases (23.9%). 125 meningiomas were discovered, with an average of 1.7 ± 1.2 meningiomas per patient (fork 1-6) and 49 patients (69%) with a single meningioma. The most frequent location of meningioma was the convexity or the region of the false in 60/125 cases (48%) and the previous region The regions of the base of the skull and the average pit in 54/125 cases (43.2%) (fig. 2). Histopathological confirmation, available for the 36 cases which were resulted (28.8 % of the meningiomas), confirmed a meningiom in all cases (22 were of grade WHO 1, 14 were grades 2 of the WHO). The patient who had a history of craniospinal radiotherapy treatment has a grade 2 meningioma.
Note from the association: WHO has changed its criteria so there are more grade 2 now than before, because WHO has revised its criteria: it increased the criteria bringing the meningiomas in grade 2, because it wants to alert that the meningiomas are ultimately less mild than it was thought before.
The number of meningiomas has not been significantly associated with the duration of the progestogen treatment (a meningioma: average duration of the treatment of 185 ± 72 months, ≥ 2 meningiomas: average duration of treatment of 216 ± 68 months; p = 0.358) or with the grade of malignancy (grade 1: average of 195 ± 73 months of treatment; month of treatment;
With regard to the progestin administered, a combination of progestogen derivatives has increased the number of meningiomas per patient (average of 2.3 ± 0.6 meningiomas), compared to cyproterone acetate (average of 1.7 ± 1.2), nomestrol acetate (average of 2.0 ± 1.8), and chlormainone acetate (average 1.6 ± 1.2, p = 0.050).
Progestin management
Following the diagnosis of MRI meningioma, progestin treatment was known for 68/71 patients (95.8%). The progestin was removed in 55/68 patients (80.9 %) and continued in 13/68 patients (19.1 %). There was an increase in the stop of progestin treatment over time : 16.7% between 1995 and 2008, 82.9% between 2009 and 2016, and 95.2% between 2017 and 2018 (p <0.001). The site: The abandonment rate of treatment varies according to the derivative progestogen used: cyproterone acetate has been removed in 40/45 (88.9 %), Chlormadinone acetate was removed in 11/13 (84.6 %), nomestrol acetate was removed in 2/7 (28.6 %), and the combination of derivatives progestins was removed in 2/3 (66.7%; p = 0.002).
Meningioma management
Management was unknown for three meningiomas (2.4 %) in two patients (2.8 %). Conservative management was proposed for 74 meningiomas (60.6 %) in 36 patients (52.2 %). Therapeutic management was made for 47 meningiomas (39.3 %) in 33 patients (47.8 %).
The clinical factors associated with therapeutic management were:
- The presence of a focal neurological deficit (50.0 % in therapeutic management against 11.4 % in conservative management, p <0.001),
- A decrease in KPS score by ≥ 20 (85 % against 95 %, p <0.001)
- and headache (50.0 % against 28.6 %, p = 0.072).
Surgical resection of 36 meningiomas was carried out in 28 patients , immediately after the diagnosis of meningioma in 25 patients and after an average of 16 ± 13.9 months with MRI monitoring in three patients.
The stereotaxic radiotherapy of 11 meningiomas (three anterior clinies, three convex, two at ponto-cerebellar angles, a cavernous sinus and a brain meningiom of the false; nine with progressive growth, two symptomatic) was carried out in five patients.
Combined treatment (surgical resection + radiotherapy stereotaxis) was carried out for meningioma in one of these patients.
No patient with grade 2 meningioma confirmed histopathologically has received radiotherapy following surgical removal.
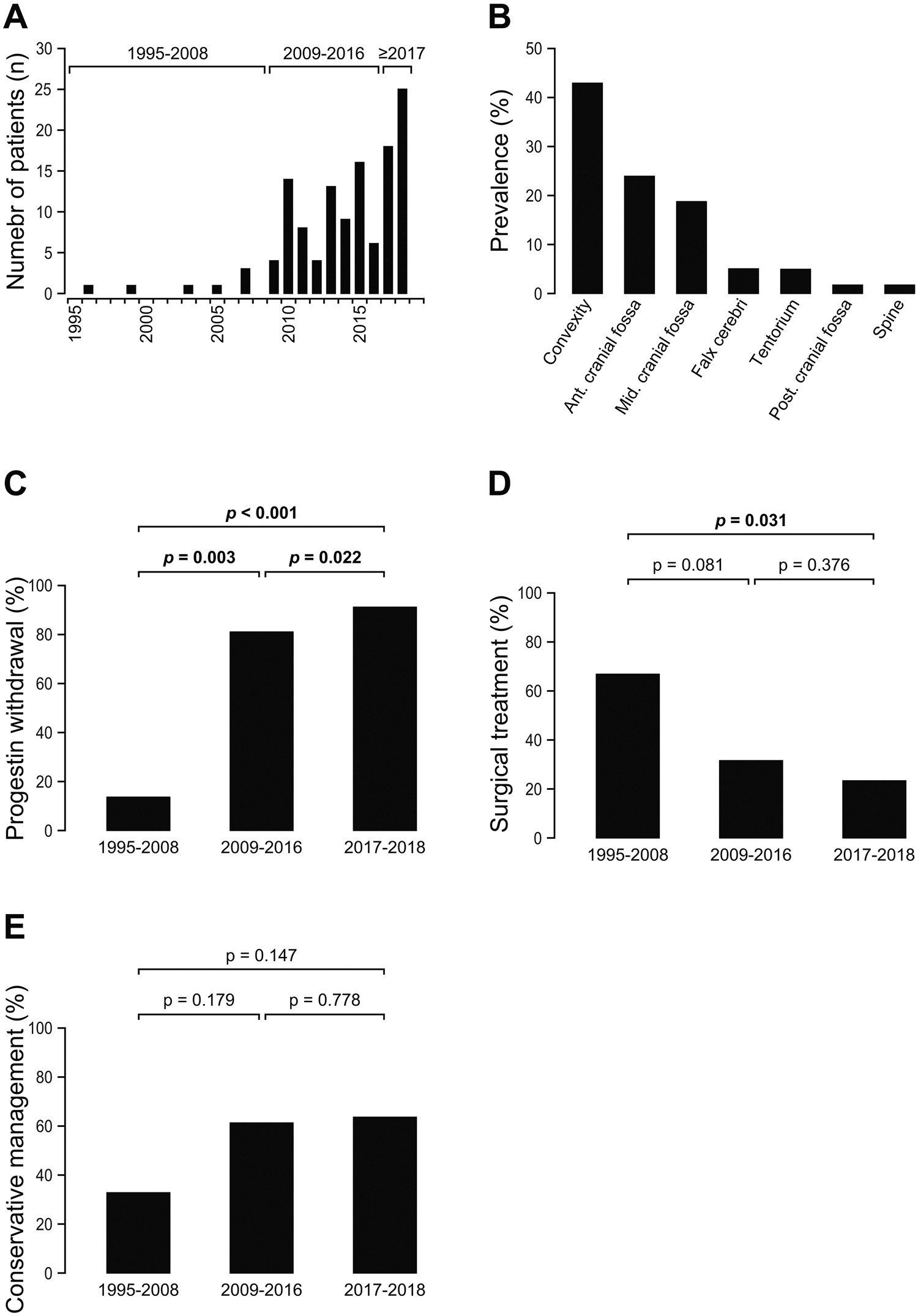
Fig. 1 A number of patients per year (N) returned to our neurosurgery service for one or more suspicious meningiomas within the framework of a long -term policy The use of progestins between 1995 and 2018. B Distribution of meningiomes location (%). c Evolution in the time of the proportion (%) of removal of progestogen after the diagnosis of one or more meningiomas between 1995 and 2018. D evolution over time of proportion (%) of surgeries the treatment of one or more meningiomas after their diagnosis within the framework of a long -term policy The use of progestins between 1995 and 2018. E evolution in time of proportion (%) meningiomas after their diagnosis within the framework of a long -term policy the use of progestins between 1995 and 2018.
The rate of treatment of meningiomas decreased (surgery and/or radiotherapy) over time: 66.7% between 1995 and 2008, 46.3% between 2009 and 2016, and 35.7% between 2017 and 2018 (<0.001) as well as an increase in the conservative management of meningiomas with a progestin treatment followed by a wean: 33.3% between 1995 and 2008, 53.7% between 2009 and 2008 2016, and 64.3 % between 2017 and 2018 (<0.001). The management of meningiomas has not varied depending on the progestin used (p = 0.452).
Table 1 Data summarized on patients, tumors, management and monitoring
characteristic of the patient and the tumor at the time of diagnosis n % male sex 0 0 0
feminine 71 100
age (years) medium ± SD (fork) 47.3 ± 9.6 (22-78)
Number of meningiomas per medium patient ± SD (fork) 1.69 ± 1.24 (1-6
) Progressive (month) Average ± SD (fork) 197.2 ± 70.8 (60-312)
Derivative of progestogen administered Cyproterone acetate 47 66.2
Chlormadinone acetate 14 19.7
Nomestrol acetate 7 9.8
Combination 3 4.3
Symptoms not 17.9
Yes 54
76.1 71.8
Yes 20 28.2
Headache NO 41 57.7
Yes 30 42.3
Epilepsy crisis NO 56 78.9
Yes 15 21.1
SCORE KPS At the time of the average diagnosis ± SD (fork) 88.5 ± 10.0 (60-100)
70 and more 70 98.6
<70 1 1.4
Location of convex meningioma 54 43.2
Falfalcine and parafalcine 6.6 An earlier or medium tank 54 43.2
Cerebellar (Tentorium) 7 5.6
Falx region and Parafalcine 6 4.8
Vertebral column 2 1.6
Ponto-cerebellar angle 1 0.8
Ponto-Cerebellar angle 1 0.8
Foramen Magnum 1 0.8
Management and monitoring data N
Progressing
Retraction No 13 18.3
Yes 55 77.5
Not available 3 4.2 Conservative meningioma 36 50.7 Treatment 32 45.1
Not available 3 4.2
Meningioma treatment Surgery 28 39.4
Stereo radiotherapy… 5 7.0
Stereo surgery/radiotherapy… 1 1.4
Postoperative
complications of the transient neurological deficit 7 25.9
ischemia 3 11.1
Infection 3 Relationship (Simpson grade) I 7 19.4 II 23 63.9
III-IV 6 16.7
WHO Malignity grade I 22 61,1
II 14 38.9
Duration of postoperative monitoring (month) Average ± SD (fork) 38.3 ± 34.4 (3-216)
Complete series 45.8 ± 41.7 (3–216)
Méningiomes treated 33.1 ± 27.3 (3–93)
Méningiomes managed in a conservative manner
KPS score during the last average monitoring ± SD (fork) 94.6 ± 9.1 (50-100)
70 and plus 49 98.0
<70 1 2.0
radiographic monitoring of the maningiomas managed conservative
(n = 59) Increased volume 3 5.1
A stable volume 40 67.8
Decrease of
Radiographic Monitoring
Méningiomes treated (n = 41) Recurrence 3 7.3 No recurrence 34 82.9
Stable residue 4 9.8
Clinical and imaging monitoring
Clinical monitoring and imaging data is illustrated in Fig. 2. Calendar of the latest postoperative imaging and follow -up campaigns for the whole series, available for 100/125 meningiomas (80.0%), was 38.3 ± 34.4 months. For the 47 meningiomas treated, the average duration of the latest postoperative imagery and clinical monitoring, available for 41/47 meningiomas (87.2%), was 45.8 ± 41.7 months.
Among the 31 meningiomas treated by surgery with a postoperative followed available, there was:
- 2 recurrences (6,5%),
- residual stable in 1 (3,2%),
- And no recurrence in 28 meningiomas (90.3%).
Of the 10 meningiomas treated with radiotherapy with an available postoperative follow -up, there was:
- a disease increase in 1 (10.0 %),
- Stable residue in 3 (30.0 %),
- and regression in 6 meningiomas (60.0%).
There have been more meningioma regressions after radiotherapy in patients under cyproterone acetate (85.7 %) than in patients under progestogen derivative (0 %, p <0.001). The management of the progestin agonist is known for meningiomas treated at 40/41 with known follow -up (97.6 %).
Among the 27 meningiomas treated for which the progestin had been removed:
- 1 (3.7 %) showed an increase in the disease,
- 3 (11.1 %) showed
- and 23 (85.2 %) showed a regression of the tumor.
Among the 13 meningiomas treated for which the progestin had been continued:
- The increase in the disease was observed in 2 (15.4%)
- And a tumor regression observed in 11 meningiomas (84.6 %) after progestin treatment.
For the 74 meningiomas managed conservatively, the average calendar of the latest postoperative imaging and monitoring exams, available for 59/74 meningiomas (79.7%), was 33.1 ± 27.3 months:
- Three meningiomas (5%) have demonstrated growth in the tumor,
- 40 meningiomas (68%) stability in growth,
- and 16 meningiomas (27%) a tumor regression.
There have been no significant differences in the growth of tumors as a function of the molecule progesive used (p = 0.230). The management of agonist progestin is known for 57/59 maningiomas managed in a conservative way, with known follow -up (96.6 %).
Among the 54 meningiomas managed in a conservative way for which the progestin had been removed:
- The growth of the tumor was present in 1 case (1.9%),
- growth stability in 37 cases (68.5%)
- and regression of the tumor in 16 cases (29.6 %).
The three meningiomas that the progestin had been prosecuted have demonstrated the presence of a growth tumor in time (100%).
Discussion
Main results
We show, in a monocentric and retrospective series of 125 meningiomas associated with a progestogen in 71 adult women, that:
(1) The number of patients with meningioma associated with a progestogen managed in our neurosurgery service has increased over time;
(2) The reason for progestin treatment was a treatment with an indication validated in a quarter of the cases;
(3) A combination of progestins considerably increases the number of meningiomas per patient compared to a single progestogen ;
(4) There has been an increase over the time of the management of meningiomas as well as an increase during the time of stopping progestin treatment;
(5) The stop of progestin treatment varies as a function of the progestin derivative used;
and (6) After removing the progestin treatment, the meningiomas associated with conservative manifolds presented a regression on monitoring imagery in 30 % of cases.
Interpretation
The meningiomas associated with a progestogy share the same imaging and the histopathological results as those of the general survey of a population.
Current data confirm the atypical level 2.
Meningiomas can be observed in the context of the use of progestins, as indicated above [22], and as observed in the general population.
The number of meningiomas per patient did not differ according to the progestin derivative used , except with a combination of progestins which was associated with an increased number of meningiomas. Although this observation is limited to three patients, which requires studies on the subject, it suggests being careful with regard to the starting indications of a second progestogen agonist in patients who already receive one.
The small number of patients for which the duration of the progestin treatment was known in our study (32.4 %) prevented from investigating the association between the duration/dose of progestin treatment and the number of meningiomas [6, 23].
Here, the real regression of meningioma after the interruption of progestogen, observed in 30 % of cases during an imagery follow -up at> 33 months, contrasts with the reports. A series of cases analyzing the effect of the use of progestins on the growth of meningiomas reported a higher rate (> 90%) of regression of meningioma after the withdrawal of progestogen [17, 18, 21]. A cohort study, focused on the use of cyproterone acetate in France [23], has demonstrated tumor regression in 50% of lesions after the withdrawal of cyproterone acetate. This observation requires multicentive and blind quantitative analyzes on long -term imaging monitoring . This study on unique centers demonstrates an increase in meningiomas associated with progestogen, referred to neurosurgery and a change in the therapeutic management of meningiomas and progestogen over time (increase in progestogen, decrease in surgical treatment of meningiomas, and increase in the conservative management of meningiomas).
The widespread use of progestogen in France since the 1980s, including the out -of -indication use, can explain the increase in the number of meningiomas associated with progestins by cumulative exposure to progestogen over time. In addition, the increase in the number of meningiomas associated with progestins over time can be linked to progress in medicine and increase knowledge relating to this specific condition. Progestic prescribers seem to be more aware of the risk of progestin meningiomas thanks to the increase in the number of publications concerning the relationship between progestin and the growth of meningiomas since 2008 [3-10, 14, 18] illustrated by an increase in meningioma diagnostics by mRI screening over time.
The new imaging screening policy proposed for all patients receiving progestogen derivatives, implemented in France in October 2018 [24, 25], will probably increase the number of meningiomas associated with progestins in the years to come. In addition, we observed that 73% of patients received a progestogy without approved indication. In France, the recommendations of the National Agency for Medicines and the Health Products Safety highlight the need to check the right use and assess the benefit/risk ratio for each prescription of cyproterone acetate [24]. This effort will probably help reduce the number of meningiomas associated with cyproterone acetate in the future.
It should be noted that the change observed in the management of progestins has varied over time depending on the progestogen agonist: cyproterone acetate has been more often removed and nomestrol acetate has been removed less than other progestogen derivatives. The prescribers must be advised concerning the side effects of each progestogen and the usefulness of its withdrawal in the case of meningiomas, keeping in mind the potential harmful effects of a brutal stop in hormonal diseases.
In this series, the treatment decision based on the presence of clinical signs or neurological deficits is reduced in the KPS score, which was more often surgical and patients with fortuitous diagnostics of meningioma were more often treated with conservative management.
We have stressed that 38.4 % of meningiomas associated with progestogen and 46.5 % of patients were treated surgically, which suggests that these meningiomas do not systematically have an indolent course and these patients are exposed to the risk of postoperative complications linked to the operation. The feasibility of a conservative management, whatever the progestin derivative used, in the absence of neurological handicap, is confirmed by current data.
To find out more: studies will assess the impact of conservative management for meningiomas associated with progestogen and with deficiencies. In addition, the relevance of the management of osteomeningioma associated with a progestogen, where the regression of the bone component of the tumor is unlikely after the stop of progestogen , remains to be treated. These observations should help advise women who have just had diagnosed meningiomas which are under progestin treatment that, although these tumors can progress, the need for surgery is rare.
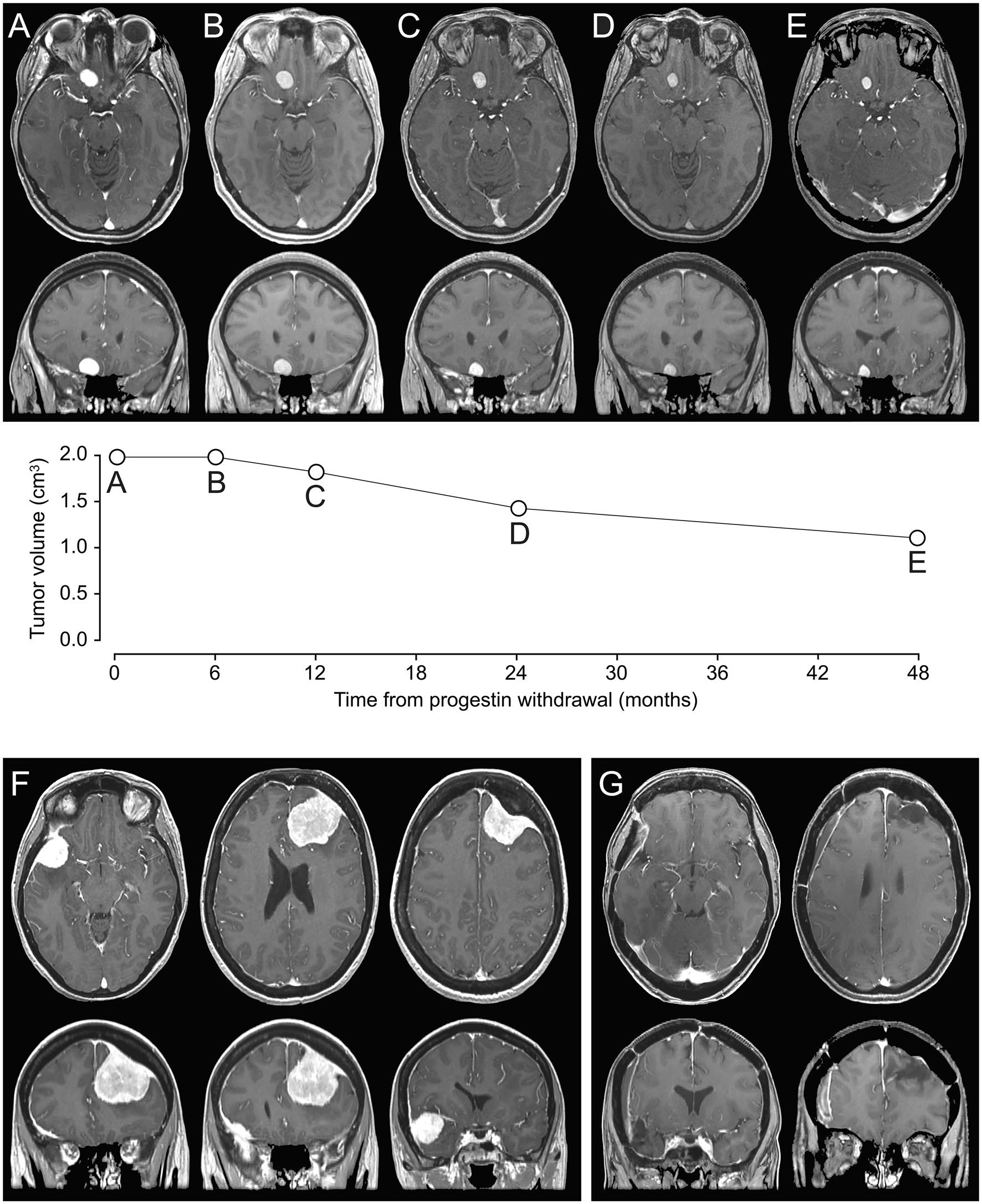
Fig. 2 AE a 34 -year -old woman with history of acne treated with cyproterone acetate for 9 years. After the diagnosis of a suspicion of Méningioma Frontoal Right on a first MRI carried out for headache, conservative management with removal of the progestogen was continued. The quantitative evaluation of sequential monitoring MRI (A, initial MRI; B, postoperative MRI at 6 months; C, postoperative at 1 year MRI; D, MRI 2 years after the operation; E, MRI 4 years after the operation) demonstrated the regression of suspected meningioma. Acetate, followed by chlormadinone acetate for 10 years. Left frontal meningioma and a right spheno-orbital osteomaning were diagnosed following a first epilepsy crisis. The progestogen was withdrawn and the two meningiomas were carried out separately at 6 months per interval. Histopathological analyzes confirmed two meningiomas of grade 2. After the operation, there was no recurrence of epilepsy attacks and antiepileptic therapy was interrupted six months after the second surgery (F: preoperative MRI, G: MRI 3 years after the operation).
Limitations
These results must be interpreted with caution, given the exploratory and retrospective design of the study, as well as missing data. We recognize the biases induced by the inclusion of each dural lesion discovered in MRI with the imaging characteristics of a meningioma, which were then called "meningioma". However, all the dural lesions operated have been confirmed as meningiomas by histopathological analyzes. We recognize the biases induced by the patients who are addressing neurosurgery themselves. It is possible that small and asymptomatic meningiomas, managed by general practitioners, gynecologists or endocrinologists, have not been seized. This could explain the high level of symptomatic meningiomas and surgically treated in this study. In addition, the relatively short monitoring below the commonly used standard of 5 years and the lack of data concerning the duration of the progestogen processing and the cumulative dose of progestin has prevented detailed analyzes of the real impact of progestin on the evolution of meningiomas, including the number of meningiomas. The question of the impact of conservative management strategies of meningiomas associated with a neurological impairment has not yet found an answer since we have no meningiomas associated with a neurological impairment in the conservative management group. However, previous reports have suggested the effectiveness of conservative management in such cases [19]. Finally, we did not carry out a central neuropathological examination blind to assess the degree of malignancy of meningiomas according to the current classification of WHO 2016 and we lack a search for the immuno-expression of the receptors of progesterone and estrogen . Changes in the histopathological classification of meningiomas according to the different versions of the WHO classification from 2000 to 2016 [26-28] can explain the atypical meningiomas of grade 2.
Consequently, the practical management of meningiomas associated with a progestogen remains to be confirmed by subsequent prospective multicentric surveys with quantitative evaluations of the changes in meningiomas during progestin treatment and after stopping treatment.
Generalization of results
This study, with a lack of detailed information concerning the use of progestins, underlines the need to normalize more data collection on essential information concerning progestin processing. Such a collection of prospective data has been started in our institution since October 2018 for all patients who have received progestin derivatives. In addition, we have now offered targeted and easy MRI access for patients under progestin treatment to facilitate screening in accordance with the recent ANSM [24]. These future data will improve our knowledge of the natural history of the meningomes associated with the progestins and their management.
Conclusion
In a monocentric series of 125 meningiomas associated with a progestogen in 71 adult women collected between 1995 and 2018, the number of patients with meningioma associated with progestogen has increased over time. Conservative management, including stopping progestin treatment, has developed over time with promising results in terms of efficiency and safety.
Author contributions hm, ts, jb, and jp the data collection. HM, ts, mz, ar, jb, and jp the data analysis. HM, ts, mz, ar, jb, gzb, me, ate, ed, ep, fc, pv, gpb, co, and jp the data interpretation.
HM, JB, SP, and JP Wrote the Report. HM, TS, Mz, Ar, JB, Gzb, Me, ATE, ED, EP, FC, PV, GPB, CO, SP, and JP Review and Approved the Paper.
Data Availability Anonymized Data will be Shared Perquet from Any Qualified Investigator.
Compliance with Ethical Standards
Conflict of Interest the Authors Declares that they have no conflict of interest to disclose.
Consent to participate the requirement to obtain informed consent was waive for this observational retrospective study accusing to french legislation.
Consent for publication all co-authors have seen and agree with the contents of the manuscript.
Ethical Approval This Study Received Required Authorizations (IRB #1: 2020/08) of the Institutional Review Board (IRB00011687). The requirement to obtain informed consent was waive for this observational
retrospective study accord to french legislation.
References
1. Ostrom QT, Gittleman H, Truitt G et al (2018) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro-oncol 20: IV1–I86. https://doi.org/10.1093/neuon c/noy13 1
2. Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and Etiology of Meningioma. J Neurooncol 99: 307–314. https: //doi.org/10.1007/S1106 0-010-0386-3
3. SYS C, KESTELYN P (2015) Unilateral Proptosis and Blindness Caused by Meningioma in A Patient Treated with Cyproterone Acetate. GMS Ophthalmol Cases 5: 2193–1496. https: //doi.org/10.3205/oc000 027
4. Note nm, wiepjes cm, de blok cjm et al (2018) The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment. Brain 141: 2047–2054. https: //doi.org/10.1093/brain/awy10 8
5. Bergoglio mt, gómez-balaguer m, almonacid folch e et al (2013) symptomatic meningioma induced by cross-sex hormone treatment in a male-to-tomale transsexual. Endocrinol Nutr 60: 264–267. https://doi.org/10.1016/j.endon U.2012.07.004
6. Gil M, Oliva B, Timoner J et al (2011) Risk of Meningioma Among Users of High Doses of Cyproterone Acetate As Compared With The General Population: Evidence from
Population Meningioma Among Users of High Doses of Cyproterone Acetate. BR J Clin Pharmacol 72: 965–968. https: //doi.org/10.1111/j.1365-2125.2011.04031 .x
7. Mancini I, Rotilio A, COATI I et al (2018) PRESENTATION OF A MENINGIOMA in a transwoman after nine years of cyproterone acetate and estradiol intake: Review. Gynecol Endocrinol
34: 456–459. https: //doi.org/10.1080/09513 590.2017.1395839
8. Alderman CP (2016) Probable Drug-Related Meningioma Deteted During the Race of Review Services. CONSULT PHARM 31: 500–504. https: //doi.org/10.4140/tcp.n.2016.500
9. CEA-Soriano L, Blenk T, Wallander Ma, Rodríguez Lag (2012) Hormonal Therapies and Meningioma: Is There A Link? Epidemiol cancer 36: 198–205. https: //doi.org/10.1016/j.canep .2011.08.003
10. Schmutz JL (2018) Cyproterone Acetate and Meningioma: The Latest Findings. Ann Dermatol Venereol 145: 390–391. https: //doi.org/10.1016/j.annde r.2018.04.001
11. Neumann F, Töpert M (1986) Pharmacology of Antiandrogens. J STEROID BIOCHEM 25: 885–895. https: //doi.org/10.1016/0022-4731 (86) 90320 -1
12. Druckmann R (2009) Profile of the Progesterone derivative chlormadinone Acetate -Upharmocodynamic Properties and Therapeutic Applications. Contraception 79: 272–281. https: //doi.org/10.1016/j.contrace.2008.10.017
13. Mueck ao, Sitruk-waare R (2011) Numegestrol Acetate, A novel Progestogen for oral contraception. Steroids 76: 531–539. http://doi.org/10.1016/j.steroids.2011.02.002
14. Galarzari R, Galarza M, Gazzari G (2007) Growth of a meningioma in a transsexual patient afterrogen --progestin therapy. N Engl J Med 357: 2411–2412. https: //doi.org/10.1056/nejmc 07193 8
15. Shimizu J, Matsumoto M, Yamazaki E, Yasue M (2008) Spontaneous regression of an asymptomatic meningioma associated with discontinuation of progesterone agonist administration. Neurol Med Chir (Tokyo) 48: 227–230. https: //doi.org/10.2176/nmc.48.227
16. Gonçalves AMG, Page P, Domigo V et al (2010) Abrupt regression of a meningioma after Discontinuation of Cyproterone Treatment: Fig. 1. AM J Neuroradiol 31: 1504–1505. https: //doi.org/10.3174/ajnr.a1978
17. Cebula h, Pham TQ, Boyer P, Froelich S (2010) Regression of meningiomas after Discontinuation of Cyproterone Acetate in A Transsexual Patient. Acta Neurochir (Wien) 152: 1955–1956. https://doi.org/10.1007/s0070 1-010-0787-2
18. Bernat al, oyama K, Hamdi S et al (2015) Growth Stabilization and regression of meningiomas after discontinuation of Cyproterone Acetate: A Case Series of 12 Patients. Acta Neurochir (Wien) 157: 1741–1746. https: //doi.org/10.1007/s0070 1-015-2532-3
19. Bernat A, Bonnin S, Labidi M et al (2018) Regression of Giant Olfactory Groove Meningioma and Complete Visual Acuity Recovery after Discontinuation of Cyproterone Acetate. J Ophthalmic Vis res
13: 355. https: //doi.org/10.4103/jovr.jovr_21_17
20. Botella C, coll G, Lemaire JJ, Ithum B (2015) intracranial meningiomas and prolonged use of cyproterone acetate in conventional dose in women: about two cases of tumor regression after stopping treatment. Neurosurgery 61: 339–342. https: //doi.org/10.1016/J.Neuch I.2015.05.002
21. PASSERI T, Champagne Po, Bernat Al et al (2019) Spontaneous regression of meningiomas after interruption of numegestrol acetate: a series of three patients. Acta Neurochir (Wien) 161: 761–
765. Https: //doi.org/10.1007/s0070 1-019-03848 -x
22. Roux a, Tauziede-Spaciat A, Zanello M et al (2020) Symptomatic Progestin-Induced A atypical II Meningioma. A first report. Neurosurgery. https: //doi.org/10.1016/J.Neuchi.2019.12.013
23. Cyproterone acetate (Androcur and its generics) and risk of meningiom: Publication of the full report of the pharmaco-epidemiology-Information point of information-National Agency for Medicines and Health Products. https: //ansm.sante .fr/s-informuer/point sd-information-point SD-Information/acetate-de-Cyproterone-Androcur-et-Ses-Géneriques-Etrisque-de-Meningiome -Publication-du-Rapport-Complete-de-L-Etude-de-Pharmaco-Epidemiology-Point-Information. Accesed 10 May 2020
24. Androcur and generics (Cyproterone acetate, 50 mg and 100 mg) and risk of meningioma: ANSM publishes recommendations for patient care - Information sign - Ansm: National Agency for the Safety of Medicines and Health Products. https: //ansm.sante .fr/S-Infor Mer/Point SD-Information-Point SD-Information/Andro Cur-et-Generiques -Actate-de-Cypro Teron E-50-MG-ET-1200-MG-ET-RISQUE E-De-Menin Giome-L-ANSM-PUBI E-RES-RECOM
TIONS nts-point -D-Infor MATIO n. Accesed 9 May 2020
25. Lotényl/Lotéran and Generics: Preliminary recommendations following confirmation of the Méningioma sur-risk-Information point-yearsm: National Agency for the Safety of Medicines
and Health Products. https: //www.ansm.sante .fr/S-Informer/point SD-Infor Matio n-Point SD-Information/Lutenyl-Luteran-et-Generiques -Recommandations -Preliminaries-Suite -A-Laconfirmation-du-sur-Risque-de-Meningiomé -Point -D-Information. Accesed 18 Nov 2020
26. Kleihues P, Cavenee WK, International Agency for Research on Cancer (2000) Pathology and Genetics of Tumours of the Nervous System. Iarc Press, Lyon
27. Louis Dn, Ohgaki H, Wiestler Od et al (2007) The 2007 Who Classification of Tumours of the Central Nervous System. Acta Neuropathol (Berl) 114: 97–109. https: //doi.org/10.1007/s00401-007-0243-4
28. Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol (Berl) 131: 803–820. https://doi.org/10.1007/s0040 1-016-1545-1
Publisher's Note Springer Nature Remains Neutral With Regard to
Jurisdictional Claims in Published Maps and Institutional Affiliations.
Note from the AMAVEA association on this study:
This study relating to 125 meningiomas in 71 women over 23 years old , in a hospital, there the Hospital Sainte-Anne du GHU Paris is essential to update and understand several factors that question:
- Increase in the number of cases over time
- Less than 30 % of indications validated for treatments (treatment S hormonals prescribed Hoars AM in 70 % of cases)
- Increase in the cessation of treatments after diagnosis of meningioma over time (doctors still learn)
- Increase in the attitude of simple surveillance over time.
- On MRI monitoring, decrease in meningiomas after stopping in 30% of cases, stability in 68% and continuation of growth in 2% of cases (we are far from the 60% decrease reported by Lariboisière).
- Need for treatment (surgery, radiotherapy) in 47% of patients (with the risks and consequences of surgery). We are far from the term "Benin" used constantly for these meningiomas by princural gynecologists and by the press!
- Unknown long -term effects
- Responsibility of the necessary doctors, sanctions against doctors who prescribe out of AMM by bringing the lives of patients into play?
Study taken up here by Neurodiem: https://www.neurodiem.fr/news/progestin-associated-meningiomas-can-be-deped-by-by-bish-discontinuing-treatment-6g0t3almzktl Knpkka0xoa? Utm_medium = Newsletter & Utm_source = Neurodiem & Utm_campaign = nd_fr_nl_weekly_04_02_2021 & utm_content = CSS%7c3%7c7
Link on pubmed https://pubmed.ncbi.nlm.nih.gov/33449307/
Find our other articles here




